Public health, explained: Sign up to receive Healthbeat’s free national newsletter here.
For moms like Elisabeth Bates and Ashlee Johnson, the decision to feed their babies ByHeart infant formula was driven by necessity and influenced heavily by words like these on the products’ labels:
“Closest to Breast Milk”
“Made with Organic Grass-Fed Whole Milk”
“Certified Clean Ingredients”
“Purity Award”
Bates, a pediatric nurse practitioner in New York City, said she wanted something better for her son than your average baby formula.
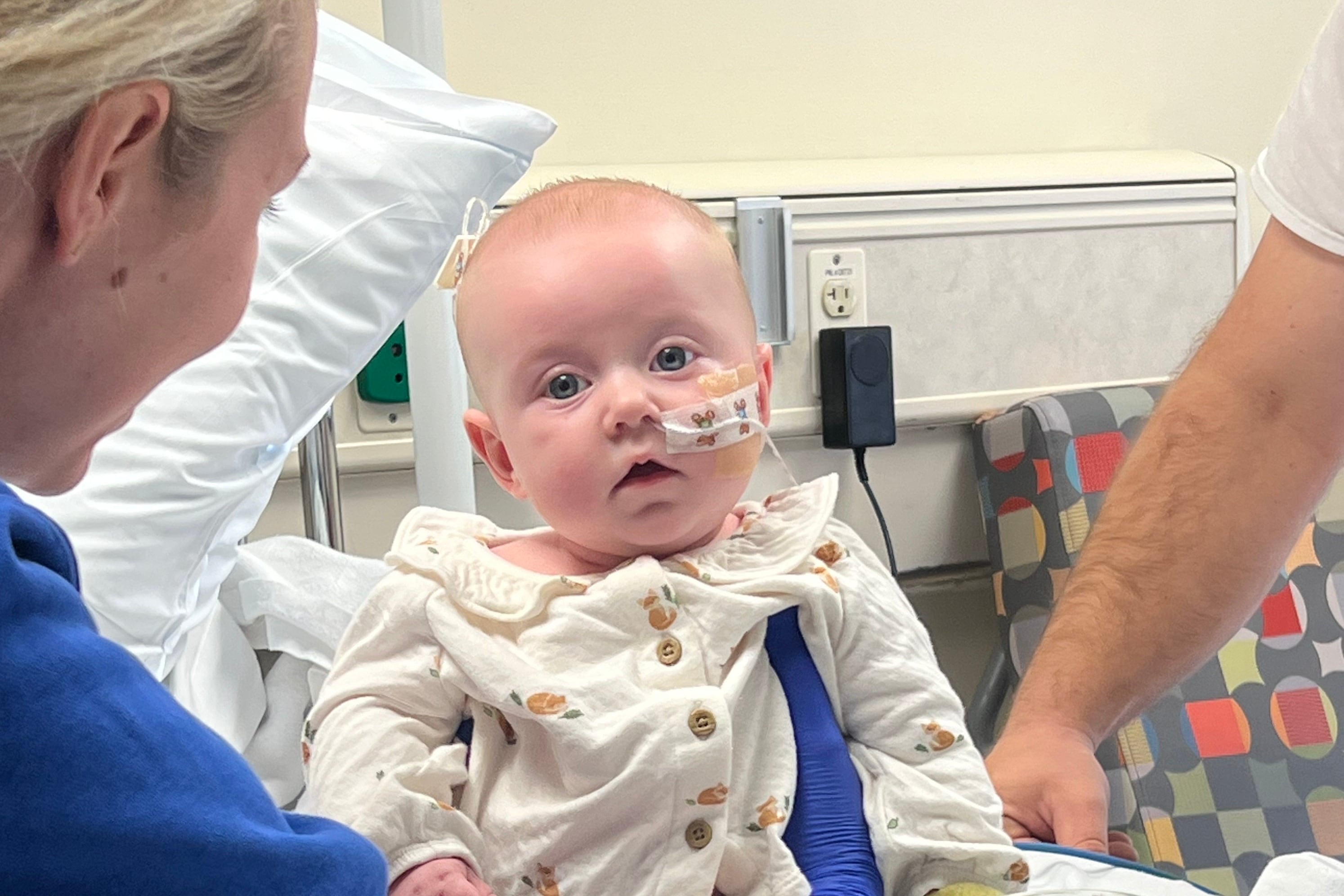
Instead – like parents across the country – Bates has been on “high alert” scrutinizing 7-month-old Beau for signs of infant botulism and counting down the 30 days until he is safely beyond the period when any botulism bacteria spores he may have consumed before ByHeart’s recall could still germinate inside his body.
“Seeing that label, you as a parent, you just kind of automatically trust that some higher body gave them this kind of certification,” said Bates, who regrets stopping breastfeeding and giving her trust to the product. “There is a big feeling of guilt.”
So far, Beau remains healthy.
ByHeart’s recall of all of its formula products last month amid a growing nationwide outbreak of infant botulism has shaken the trust of moms and caregivers – not just in ByHeart’s formula, but also in formula made by other brands, moms from across the country told Healthbeat.
Nearly a month has passed since regulators and state health officials issued the first public warnings on Nov. 8 that ByHeart’s baby formula was suspected of fueling an increase in infant botulism hospitalizations across the country. Yet as the weeks have gone by, they’ve released little information about how botulism spores could have gotten into ByHeart’s formula – such as whether the source is likely inside a ByHeart manufacturing facility or in an ingredient used to make the companies’ formula.
This lack of information, the moms said, is not reassuring for families already on edge after making a hasty switch to different companies’ formulas in the wake of the ByHeart recall.
Johnson, who lives in Bremerton, Wash., said she worries about the potential for botulism contamination in the new formula she’s feeding her 5-month-old son, Elio.
“Even with the formula we’re using now, if he has an off day, I’m like: Is there something wrong with this one?” Johnson said. “It’s supposed to be top of the line, good stuff from a reputable company. And you are still worried about it. What if there’s another recall for this one?”
Bates said even her friends who never fed ByHeart formula are worried: “If this can happen to one formula, they’re also wondering: Could my formula be next?”
Few details released about how ByHeart formula became contaminated
At least 39 infants who were fed ByHeart formula have been hospitalized in 18 states with suspected or confirmed infant botulism, according to the Food and Drug Administration’s outbreak update on Wednesday. The dates these infants first started showing signs of illness ranged from Aug. 9 to Nov. 19.
ByHeart announced a small, initial recall of two batches of its formula on Nov. 8, then recalled all of its products on Nov. 11 after more babies were hospitalized.

Infant botulism is caused by babies ingesting spores of the bacterium Clostridium botulinum, which can colonize their large intestine. Once inside the body, it can take up to 30 days for the spores to activate and produce a paralyzing toxin in the child’s intestine, then start causing symptoms, such as constipation, difficulty feeding, and signs of muscle weakness, such as a floppy head.
The FDA and the Centers for Disease Control and Prevention have said little about their investigation into how botulism bacteria got into unopened containers of ByHeart infant formula. They have previously said they have not identified any other infant formula brands that pose a risk.
While ByHeart officials initially had emphasized “there is no historical precedent of infant formula causing infant botulism,” the company has since said that its own recent testing has detected botulism bacteria in five of 36 samples of unopened formula packages.
“Based on these results, we cannot rule out the risk that all ByHeart formula across all product lots may have been contaminated,” the company said late last month. “We continue to be focused on finding the root cause, through a rigorous audit of every step of our product development chain, from suppliers and raw ingredients, through to packaging and transportation.”
ByHeart did not answer questions from Healthbeat about what has been done to investigate each of those manufacturing steps and examine the component ingredients that were used to make the contaminated products.
Officials at the FDA and the CDC also did not answer questions from Healthbeat about what their investigation has found in the search for where and how botulism spores got into the product.
“FDA’s investigation, including onsite inspections, is ongoing to determine the point of contamination,” the agency said in an outbreak update last week that provided no details about what those recent inspections have found at ByHeart’s manufacturing plants or at any other companies that supplied the individual ingredients used to make ByHeart’s formula.
The FDA released redacted copies of inspection reports for three ByHeart manufacturing facilities from February and March 2025, and January 2024. The reports show manufacturing and contamination problems, some of which have been previously reported by Healthbeat and other news organizations.
But the FDA has released nothing about what its inspectors and investigators have found during the past month – in the wake of the infant botulism outbreak – as they have examined ByHeart’s manufacturing facilities, and potentially also the facilities of other companies supplying ingredients to ByHeart.
ByHeart told Healthbeat this week that “no observable links” have been found between the FDA’s facility inspection findings and the botulism bacteria that tests have detected in the company’s formula.
‘No signals’ other brands of infant formula are at risk, California officials say
The outbreak was initially identified by the California Department of Public Health, which noticed an increase in requests for a drug it makes to treat infant botulism. The drug, called BabyBIG, is the only FDA-approved treatment for infant botulism. California’s health department also serves as the nation’s infant botulism program.
The FDA has said it was alerted on Nov. 6 that California officials had identified a cluster of infant botulism cases linked to ByHeart’s formula.
CDPH told Healthbeat Tuesday evening that among the 118 infants nationwide treated for infant botulism with BabyBIG between Aug. 1 and Nov. 30, the only cases with a common source of exposure are those that have been linked to ByHeart’s formula.
“To date, there have been no signals that other infant formulas have been affected,” the department said in an email.
“It is usually difficult to pinpoint a root cause of any infant botulism case,” the department said. “Infant botulism is a very rare disease, and cases typically occur sporadically.”
CDPH noted that investigations and testing over many years indicate that most infant botulism patients become infected by simply swallowing microscopic dust particles containing spores. Honey is also a known and avoidable source of botulism bacteria spores.
The FDA and CDC did not respond to questions from Healthbeat about the potential for other products to be contributing to the recent rise in infant botulism cases.
Could contaminated milk or another ingredient be the source of botulism spores?
While ByHeart has said botulism bacteria has not been among the pathogens routinely tested for across the formula industry, formula maker Mead Johnson – whose brands include Enfamil – told Healthbeat it has tested its products for botulism contamination for years.
“Every lot of ingredients containing dairy undergoes testing for possible infant botulism contamination during the raw material phase because these bacteria can occur naturally in soil and farm environments, and we aim to mitigate risk right from the start,” Mead Johnson said in an email on Tuesday. “We also conduct routine clostridium and spore testing to ensure the quality and safety of our finished powdered products.”
The testing has not showed any evidence of infant botulism contamination, according to the company’s review of readily available testing records.
“To the best of our knowledge, there had never before been a confirmed case of botulism linked to infant formula. We are closely monitoring the recent competitor recall and look forward to understanding the circumstances behind it,” Mead Johnson said in its statement.
Formula maker Bobbie, which like ByHeart markets itself as a premium organic, whole milk formula, recently announced it is increasing testing of its facilities and products.
As of November, in the wake of ByHeart’s recall, Bobbie has added to its existing testing protocols a type of test that can identify potential spore-forming bacteria, including botulism bacteria. This kind of sulfite-reducing clostridia testing is a screening tool that can serve as an “early-warning system” that further testing is necessary if a positive result comes back, the company said.
It’s unclear what actions other formula makers are taking as a result of the ongoing ByHeart investigation.
Officials with the Infant Nutrition Council of America, an association of infant formula and toddler milk manufacturers, did not respond to repeated requests for comment since Nov. 20 about the potential implications of ByHeart’s botulism contamination for the wider formula industry. The council’s members include Abbott Nutrition, which makes Similac brand formula, and Perrigo Nutrition, which makes store-brand formulas and owns the rights to Nestlé’s Good Start brand.
Bill Marler, an attorney who has specialized in representing victims of foodborne illnesses for more than 30 years and who has filed four lawsuits against ByHeart on behalf of the families of sickened infants, told Healthbeat that manufacturing sanitation and ingredients are the primary suspects.
Marler said that ByHeart’s use of whole milk, not used by many formula makers, is a potential suspect ingredient. But the sanitation issues described in the FDA’s past inspections of ByHeart’s manufacturing plants are also concerning, he said.
“Hopefully they’re going to be able to figure out the number of kids that are sick and what likely lot they consumed from, and then start working their way back upstream,” said Marler, whose firm is based in Washington state.
David Clark, a dairy product industry consultant who specializes in infant formulas, said contaminated milking equipment is one of many potential sources of the contamination in ByHeart’s products.
Botulism bacteria can be found in the environment, stalls, and bedding on dairy farms, he said. The spores, he notes, are not killed by pasteurization. That’s why there are strict requirements for cleaning the cows’ udders and milking equipment, said Clark, whose firm Bovina Mountain Consulting is based in Englewood, Fla.
Officials with the U.S. Department of Agriculture, the federal agency responsible for animal health, did not respond to questions from Healthbeat about whether there have been any botulism outbreaks on dairy farms in the past year, whether there are any requirements for such outbreaks to be reported, and whether there are any regulations that require milk from infected cattle to be withheld from the food supply.
“Please direct these questions to the U.S. Food & Drug Administration,” the USDA press office replied in an email. The FDA also did not respond to the questions.
ByHeart formula carried ‘Purity Award’ certification from Clean Label Project
In addition to ByHeart’s manufacturing plants undergoing FDA inspections, the company’s cans and pouches were emblazoned with a certification that the formula had received a Clean Label Project “Purity Award.”
But the certification never involved any testing for pathogens like those that cause infant botulism, said Kate Stuard, a spokesperson for the Clean Label Project, a nonprofit group based in Colorado.
The project’s voluntary certification program, which manufacturers can apply to, tests a wide range of consumer products for industrial and environmental contaminants such as heavy metals, pesticides, and phthalates, Stuard said. The certification’s rules also require products be produced in facilities that maintain compliance with Good Manufacturing Practices.
The Clean Label Project recently removed ByHeart’s formulas from its list of certified products, a review of archived images of its website shows. The organization did not answer Healthbeat’s questions about when and why ByHeart’s listing was removed recently. The group also didn’t answer whether it took any actions against ByHeart’s purity certification in the wake of a previous recall in December 2022 for a different kind of bacterial contamination that prompted the FDA to issue a formal warning letter to ByHeart in August 2023.
The organization issued a general statement that “if a brand is subject to a recall, we suspend their certification pending the outcome of the investigation.” But it’s unclear what is done to alert consumers to any suspension.
ByHeart, which acknowledged the project’s practice of suspension during a recall investigation, told Healthbeat that the Clean Label Project “did not take action against ByHeart’s certification after a regulatory inspection or report. FDA inspections are a routine regulatory practice, and a core part of ByHeart’s continuous improvement program.”
The Clean Label Project last month announced it has been selected to assist with Amazon’s Compliance Fast Track Program that will allow certified brands to have automatic compliance verification with the online retailer.
Health and Human Services Secretary Robert F. Kennedy Jr. last spring announced an initiative to improve the safety and nutrition of infant formulas. But so far the program, called Operation Stork Speed, has indicated a focus on nutrition reviews and testing for heavy metals and other contaminants.
Lack of answers about source of ByHeart contamination fuels concerns among moms
For now, the biggest question for many parents and caregivers remains: How did ByHeart’s botulism contamination happen – especially in a premium product that emphasized its purity to consumers.
“What if it happens at another facility?” asked Dezirae Davies, a medical assistant and mom in Canton, Ohio. “These are our prides and joys that we’re talking about. And some people don’t have the option of breastfeeding babies. So, it’s a really important thing to find out: How did this happen and why did this occur?”

Davies said she chose ByHeart after a lot of research because she wasn’t producing enough milk after six months of breastfeeding her son, Koa. “We wanted something that was organic and more naturally made,” she said. “We wanted something that was comparable to my own breast milk.”
Koa did well on ByHeart’s formula for months until Davies got an alert on her phone last month about the recall. She remembers the horrible wave of panic as she realized that the recalled cans included one that Koa – who was by then 11 months old – had just finished.
“We talked to poison control for like an hour,” Davies said. As the family watched for infant botulism symptoms, Koa developed diarrhea from switching formulas. So they switched him to whole milk, based on his age and his doctor’s advice, and he’s now doing well, she said.
But dozens of families across the country have not been so fortunate.
Hanna Everett of Richmond, Kentucky, said her 4-month-old daughter, Piper, is among the dozens of infants who have been sickened with infant botulism after being fed ByHeart formula.
Piper, who Everett said had recently started drooling her formula out the sides of her mouth, became constipated the same weekend ByHeart announced its first recall. What hadn’t seemed anything serious, became terrifying warning signs in the wake of the recall announcement, prompting the family to rush Piper to the emergency room.
“They had to start numerous IVs, and they had to put a feeding tube in because of the muscle paralysis. They were worried she was going to aspirate,” Everett said.
“Watching them do the feeding tube and the IVs was just awful,” Everett said. “It’s hurting her. She’s screaming and crying.”
Once she started receiving the BabyBIG botulism treatment, which had to be shipped to the hospital, Piper clearly started feeling better, and she was able to return home within a few days, her mother said.
Doctors expect Piper to recover, Everett said, though she will need to receive feeding therapy to help improve her sucking and eating. “We caught hers very early,” Everett said. “We’re very thankful for that.”
Everett said picking out a new formula to feed Piper after ByHeart’s recall was incredibly stressful, but necessary because she has had difficulty producing enough breast milk.
“That was already a big decision the first time around, and I thought I made the right choice. Then it came back to hurt my daughter,” she said.
Everett’s message for the FDA and ByHeart: “They need to do better.”
“They were in trouble multiple times. I feel like the government agencies really let the ball slip on this,” said Everett, who has sued ByHeart in federal court. “It’s not something you should ever have to worry about when feeding your children.”
This article has been updated to include an update Wednesday from the FDA on the number of cases.
Alison Young is Healthbeat’s senior national reporter. You can reach her at ayoung@healthbeat.org or through the messaging app Signal at alisonyoungreports.48